Which of the following carbocations would be expected to rearrange? This question lies at the heart of understanding carbocation stability and its impact on rearrangement. Carbocations, positively charged carbon ions, exhibit varying degrees of stability based on their structure and surrounding environment.
This stability directly influences their propensity to rearrange, leading to the formation of more stable carbocations.
The stability of carbocations is governed by several factors, including the number of alkyl groups attached to the positively charged carbon, the presence of resonance structures, and the electronegativity of neighboring atoms. Primary carbocations, with only one alkyl group attached to the positively charged carbon, are the least stable and most likely to rearrange.
In contrast, tertiary carbocations, with three alkyl groups attached to the positively charged carbon, are the most stable and least likely to rearrange.
Types of Carbocations: Which Of The Following Carbocations Would Be Expected To Rearrange
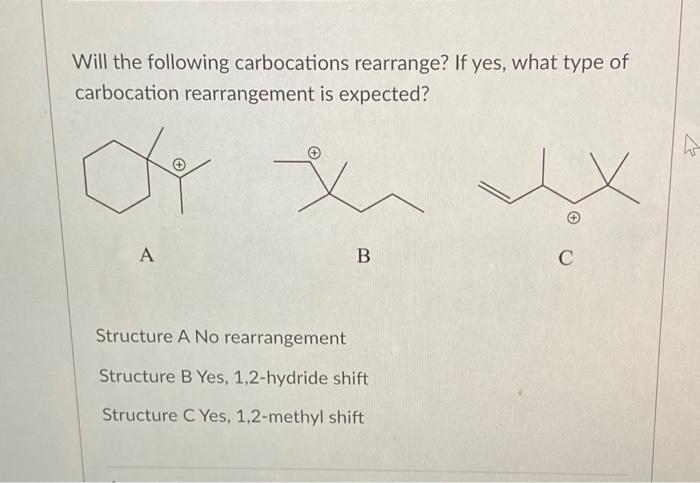
Carbocations are positively charged carbon atoms that are formed by the removal of a hydride ion from a hydrocarbon.
Primary Carbocations
Primary carbocations are formed by the removal of a hydride ion from a primary carbon atom, which is a carbon atom that is bonded to only one other carbon atom.
Secondary Carbocations, Which of the following carbocations would be expected to rearrange
Secondary carbocations are formed by the removal of a hydride ion from a secondary carbon atom, which is a carbon atom that is bonded to two other carbon atoms.
Tertiary Carbocations
Tertiary carbocations are formed by the removal of a hydride ion from a tertiary carbon atom, which is a carbon atom that is bonded to three other carbon atoms.
Stability of Carbocations
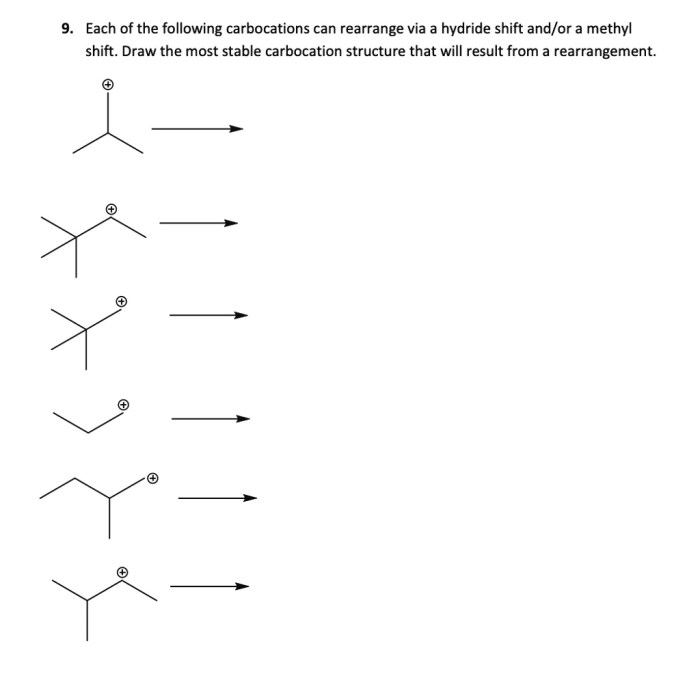
The stability of a carbocation is determined by the number of alkyl groups that are bonded to the carbocation. The more alkyl groups that are bonded to the carbocation, the more stable the carbocation.
The stability of a carbocation is also affected by the hybridization of the carbocation. Carbocations that are sp 2-hybridized are more stable than carbocations that are sp 3-hybridized.
Carbocation Rearrangements
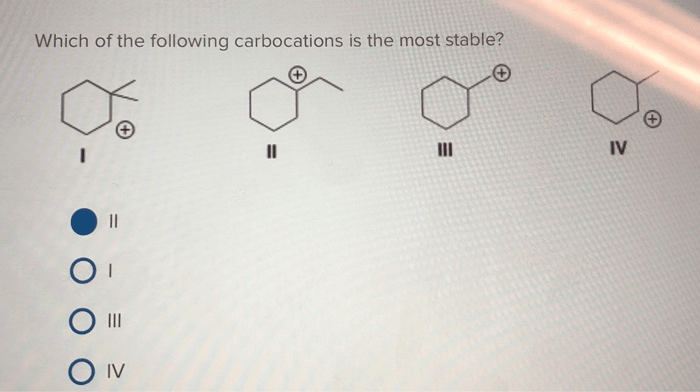
Carbocation rearrangements are reactions in which a carbocation is converted into a more stable carbocation. Carbocation rearrangements can occur through a variety of mechanisms, including:
- 1,2-hydride shifts
- 1,2-alkyl shifts
- Wagner-Meerwein rearrangements
Predicting Carbocation Rearrangements
The following factors can be used to predict whether a carbocation will rearrange:
- The stability of the carbocation
- The number of alkyl groups that are bonded to the carbocation
- The hybridization of the carbocation
FAQ
What are the different types of carbocations?
Carbocations are classified into three main types: primary, secondary, and tertiary. Primary carbocations have one alkyl group attached to the positively charged carbon, secondary carbocations have two alkyl groups attached, and tertiary carbocations have three alkyl groups attached.
Which carbocations are most stable?
Tertiary carbocations are the most stable due to the electron-donating effect of the three alkyl groups, which helps to stabilize the positive charge.
Which carbocations are most likely to rearrange?
Primary carbocations are the most likely to rearrange due to their high energy and instability.